N-CHLORO SUCCINIMIDE CAS No. 128-09-6
General Description
N-Chloro Succinimide (NCS) is a versatile and convenient reagent to chlorinate organic compounds by additions or electrophilic substitution.

Advantages over other reagents such as chlorine itself, chloramine T or sulfury chloride:
The ease of handling of the reagent itself
The mild reaction conditions of its use
The easy removal of Succinimide after the chlorination step.
Applications:
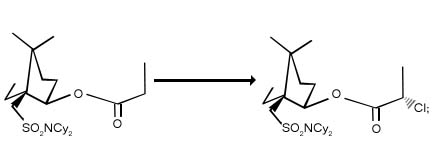
1. Chlorination in carbonyl and carbonyl compounds :
Enolates and enol ethers of ketones and esters are chlorinated in the ά -position. With suitable chiral auxiliaries present, the reaction can proceed with high diasteroselectivity.
2. Chlorination of sulfides and sulfoxides:
NCS is the best reagent to transform sulfides into ά -Chloro sulfides, which are versatile intermediates, for example for the preparation of aldehydes and ketones. Variations of these reactions involve the degradation of carboxylic acids to ketones (via ά -sulfination, reaction with NCS and hydrolysis) and the removal of the 1, 3-dithiane protective group.

3. Research with vinylic and acetylenic compounds:
Organometallic derivatives of vinylic compounds are readily converted into the corresponding vinyl chlorides
4. Chlorinations of aromatic compounds:
Electron-rich heterocycles such as thiophenes, pyrroles and indoles can be chlorinated in the ά -position; the combination of NCS and dimehyl sulfide has been used to chlorinate phenols in ά -position.
Handling:
Packing
Tariff No.
EINECS No.
TSCA
MITI
Toxicity
Safety recommendations
: PE drums of 25kg & 40kg net
: 292519
: 2048788
: not listed
: 5 - 121
: LD50 (rat, oral): 2700mg/kg
: R22, R36, R37, R38;
S22, S24, S25, S26


